White Paper
Performance of the Prodigy Voice®, Prodigy AutoCode®, Prodigy Pocket®, Blood Glucose Monitoring Systems with Prodigy® No Coding (3 Lead) Test Strips.
Overview:
All blood glucose monitoring systems available in the United States have been thoroughly tested and have met the requirements of the ISO 151971 standard and FDA Guidance Documents2. Many of these tests are conducted in the laboratory under controlled conditions with trained technicians performing the tests. These laboratory tests are very important in establishing the performance characteristics of the system. However, a clinical evaluation of the system establishes how well the system will perform and determines the accuracy of the measurements. The accuracy of self-taken blood glucose measurements was determined as part of a User Performance Evaluation using Prodigy® meters and the new Prodigy® No Coding (3 Lead) test strip.
Study Description:
The User Performance Evaluation was conducted with 150 subjects with an age range of 15 to 80 years with approximately equal numbers of males and females. Three Prodigy® blood glucose meters and three lots of Prodigy® No Coding (3 Lead) test strips were used in the evaluation. Hematocrit readings of 20% to 60% were acceptable for the study.
Procedure:
1. Subject takes capillary blood sample from finger stick and measures blood glucose concentration with Prodigy® meter and Prodigy® No Coding (3 Lead) test strip.
2. Subject takes blood sample from alternate site(s) and measures blood glucose concentration.
3. Technician takes blood sample from finger stick and measures blood glucose concentration.
4. Technician draws venous blood sample from forearm then determines hematocrit and measures blood glucose concentration from plasma by the YSI 2300 Blood Glucose Analyzer.
Results:
Capillary Blood (Finger Stick) vs. YSI Plasma Measurements
Results:
Capillary Blood (Finger Stick) vs. YSI Plasma Measurements
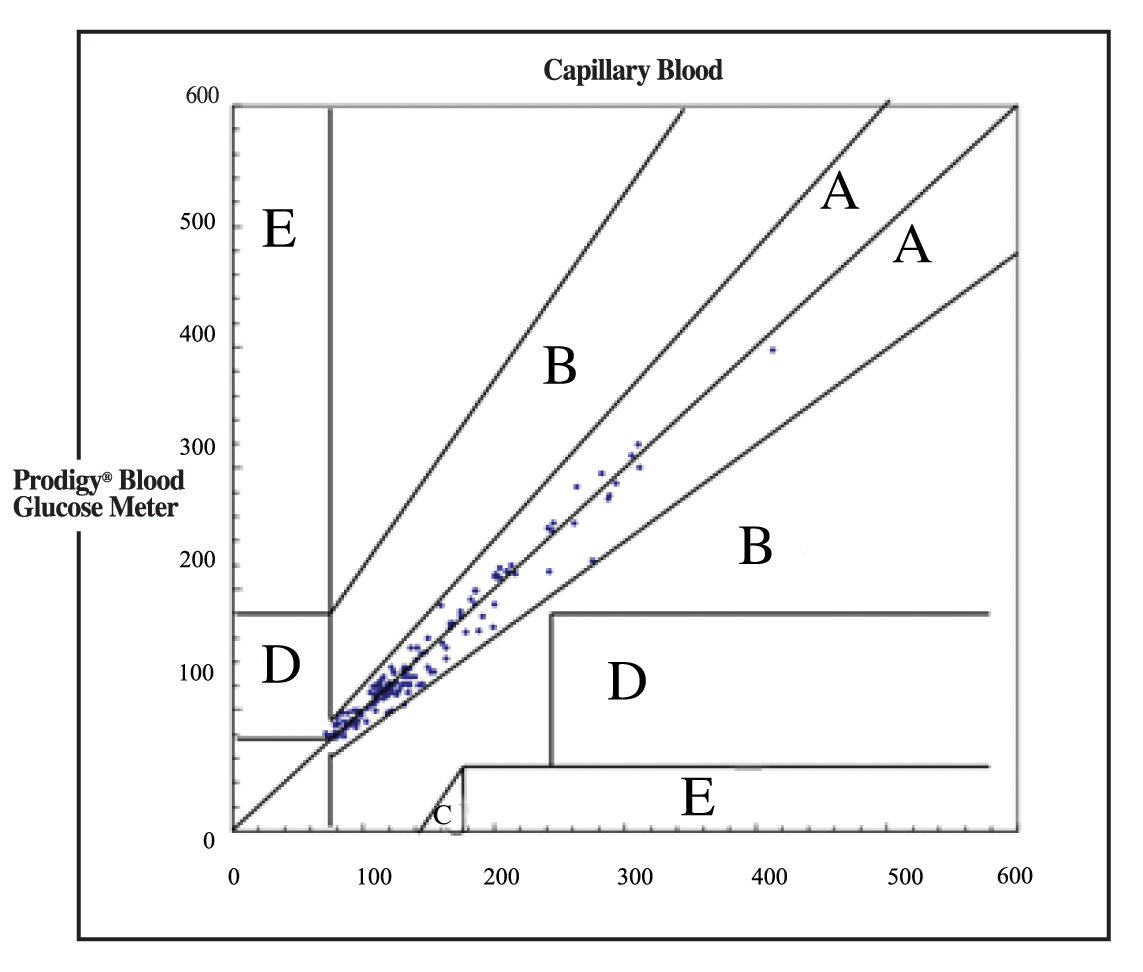
The results are displayed using the Clarke Error Grid Analysis3
One hundred percent (100%) of the measurements taken by the subjects and compared to the blood glucose concentrations measured by the YSI 2300 Blood Glucose Analyzer fall within Zone A of the Clarke Error Grid.
The Significance of the Zones in the Clarke Error Grid:
Zone A – Glucose values deviating from the reference method by less than 20% are accurate and acceptable results.
Zone B – Deviation greater than 20%, leading to benign or no treatment error.
Zone C – Resulting in over-correcting acceptable blood glucose levels.
Zone D – Dangerous failure to detection and treatment.
Zone E – Erroneous treatment error.
Conclusions:
The performance of the Prodigy® No Coding (3 Lead) test strip lots exceeds the guidelines of the ISO 15197. The Prodigy® Blood Glucose Monitoring Systems provide highly accurate results despite the effects of a diverse patient demographic, a wide range of hematocrit, differences in lots of test strips and environmental influences.
References:
1. ISO 15197 In vitro diagnostic test systems – Requirements for blood glucose monitoring systems or self-testing in managing diabetes mellitus.
2. FDA Guidance Document: Review Criteria of Portable Blood Glucose Monitoring In Vitro Diagnostic Devices Using Glucose Oxidase, Dehydrogenase or Hexokinase Methodology, February 28, 1997.
3. Clarke, WL, Cox D, Gonder-Frederick LA, et al: Evaluating Clinical Accuracy of Systems for Self Monitoring of Blood Glucose, Diabetes Care 10: 622-628, 1987.
This white paper is for informational purposes only. Requests for permission to distribute or reproduce this document must be directed by Prodigy Diabetes Care, LLC.
Prodigy Autocode® is a registered trademark. All rights reserved. CAF160 Rev3 06/10.